BUSINESS
事業案内
One-stop response for the complete pharmaceutical production equipment
We provide integrated services including design, manufacturing, and after-maintenance, focusing on preparation equipment, cell culture equipment, and inactivation equipment related to pharmaceutical manufacturing.
PRODUCTION
FLOW
生産フロー
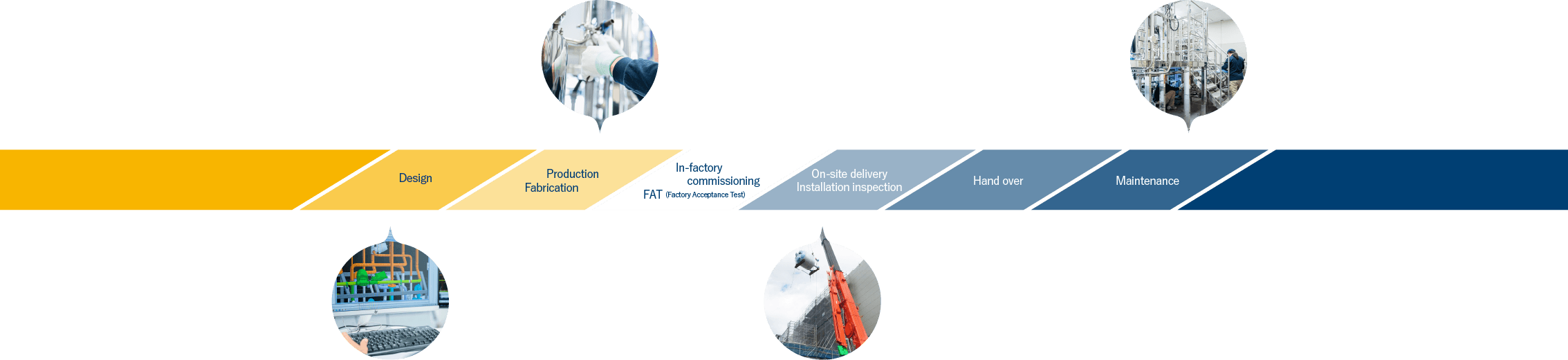

At Topsystem, each product is custom-made to meet customer requirements. In the beginning, our sales staff will propose a plan in line with customer requirements, and work with the customer from the basic design of the equipment. Then, we provide an integrated system from design to manufacturing, inspection, validation, and after-sales maintenance. We provide an environment for tooling up the producing pharmaceuticals with one-stop system.
Sales
Sales in leading edge of the pharmaceutical industry
Every year, the pharmaceutical industry develops various kinds of new drugs and invests in facilities . In the situation, our sales personnel obtains information promptly and proposes solutions which meet the customers’ needs. The first priority is communicating with customers, so we always cultivate a knowledge of technical terms and the knowledge required to understand the customer’s needs.
In recent years, we have also been focusing on overseas business, and are engaged in a wide range of aggressive sales activities both in Japan and overseas.

Design・Fabrication
Design・Fabrication
We take into account operability, maintainability, and future expansion, and use our accumulated know-how from past achievements in our design process. Process design, mechanical design, Electrical instrumentation design, system design are aligned to provide better proposals in terms of both hardware and software. For manufacturing, we provide integrated manufacturing services, including pipe fabrication , Frame and stage fabrication, control panel manufacturing, skid fabrication and electrical instrumentation work. We take full responsibility for installation and on-site inspection at the customer’s factory.
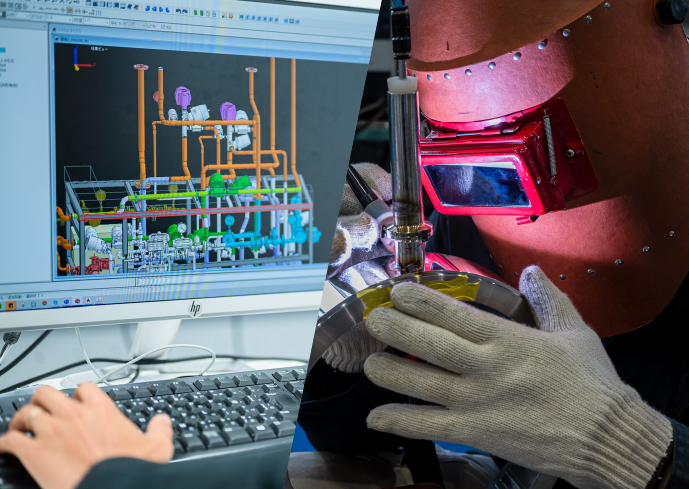
Inspection and Validation Services
Inspection and Validation Services
Since pharmaceutical products are used in the human body, strict quality control is required for pharmaceutical manufacturing equipment. Using a high-performance endoscope, we thoroughly check the inside of the piping. In addition to the inspection of equipment, piping units, and electrical instrumentation, we also conduct FAT (Factory Acceptance Test), IQ (Installation Qualification), calibration, commissioning, and OQ (Operational Qualification) at the customer’s factory. Based on these records, we carry out validation work, which is indispensable for manufacturing equipment that complies with PIC/S-GMP. Validation involves documenting the production process of the equipment itself, from design to hand over, and proving that the equipment is in accordance with the User Requirement Specification (URS).

Maintenance
After-sales service
We propose the most appropriate maintenance program for your equipment. To ensure safe and stable operation of equipment , we perform inspections of equipment itself, Operation check of power equipment, and calibration of instrumentation.

A Nationwide Network of Partner Companies
We have a network of partner companies throughout Japan, from Hokkaido in the north to Kyushu in the south. We provide speedy maintenance services to customers all over Japan.

Periodic inspection and repair of Preparation Equipment
We suggest the replacement of parts that are subject to wear and tear based on the customer’s usage conditions such as gaskets for equipment and valves that are in constant operation.